The content is published under Creative Commons Attribution 4.0 International license.
Reviewed Article:
Historical Techniques: Cold Gilding
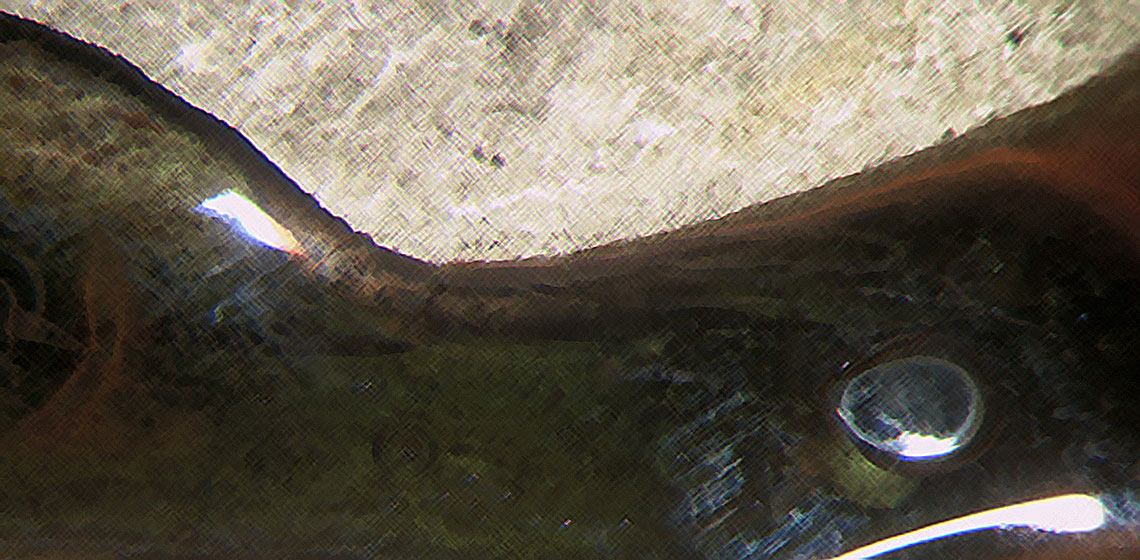
An historal technique of goldplating, described in 18th century literature, was reproduced. This cold-plating technique uses salts of gold, produced by dissolving gold in aqua regia. these salts are then rubbed onto a silver surfaces. Test prove that with this technique a thin goldlayer can be created on silver, though due to the small thickness it can not stand much wear.
Introduction
The reproduction of an old technique is part of the 5th semester of the study to become a metal conservator at the Netherlands Institute for Cultural Heritage (ICN). The technique should be based on treatises and other ancient sources. By reproducing and further research on the results of the reproduction, it is possible to understand more about objects from the past. Problems with objects can be better understood if we know more about how they were made. Reproduction of old techniques may also help with finding new conservation or restoration method's for treating of ancient objects.
During the “Bronze-week” in the fall of 1999 the technique of cold-gilding is was reproduced. This technique makes use of salts of gold to create a goldplating on metal surfaces. Although this technique is not extensively described in modern literature, some recipes can be found in 18th century literature. The Dutch treatise of Van Laer, Weg Wijzer voor aankomende goud- en zilversmeden (Van Laer 1721, 166), gives an accurate description of how this technique can be used. Another recipe can be found in Höhne & Rösling`s Kupferschmiedhandwerk (Kupferschmiedhandwerk 1839, 371).
There was little to be found in 20th century literature. Taylor (Taylor 1963, 56) only gives a short description. A recipe based on gold chloride [AuCl or AuCl3] is to be found in Schwahn's Workshop Methods (Schwahn 1960, 109). No other recipes were found. There are recipes on replacement gilding though, a technique, chemically similar to the 'rubbed-on' gilding technique, but which is being done by dipping the object in a liquid.
Making use of the ICN's database of historical resources, I found a hand-written document by an anonymous I.I.H. (I.I.H. 1700-1784, 37), of which copies are available at the ICN library.
Theory
This paragraph gives a theoretical explanation for the chemical process of the cold gilding technique. A description of this technique can be found in Experimentsand Appendix A. The chemical mechanism is similar to the more modern replacement gilding, which is done by dipping the object in a solution of salt (Schwahn 1960, Diebener 1962)
A mixture of concentrated nitric acid and hydrochloric acid (Aqua Regia) forms nitrosylchloride and chlorate:
HNO3 + 3HCl à NOCl + 2Cl + 2H2O
This heavy acid combination makes it possible to dissolve gold. The van Laer`s recipe (See Appendix A) only uses nitric acid and common kitchen salt (NaCl). Probably the following reaction occurs:
HNO3 + H2O ßà H3O+ NO-3
Followed by:
2H3O+ + NO-3 + CL- àNOCl + 3H2O + ½O2
If gold is dissolved in this acid, tetrachloric-gold (III)-acid (H[AuCl4].2H2O) is formed when the liquid evaporates. The water of crystallisation will be driven off by heat according to the recipes, but the H[AuCl4] is very hygroscopic, and will probably take up some water after burning of the linen (see recipes).
Using the old recipes, a pieco of linen is soaked in the gold-acid solution. The linen is left to dry and then burned. The ashes are rubbed onto aea9slver) surface using a cork. hereby plating the silver with a thin layer of gold.
The half-reaction for the reduction reaction which forms gold, has a electrochemical potential of 0.98 V:
AuCl-4 (aq) + 3e- ßàAu (s) + 4Cl- (aq)
Possibles half-reactions for oxidation at a silver or copper-alloy surface are:
| Ag (s) | <- -> | Ag+ + e- | E=-0.80 V |
| Ag(s) + Cl- | <- -> | AgCl(s) + e- | E=-0.22 V |
| Cu(s) | <- -> | Cu+ + e- | E=-0.52 V |
| Cu(s) | <- -> | Cu2+ + 2e- | E=-0.34 V |
We can conclude that a possible mechanism for the cold gilding is:
nAuCl-4(aq)+ nMe(s) ßànAu(s) + nMeCl + nCl- (aq) (Me=metal)
Most reasonable are the half-reactions no. 2&4. However, other reactions will occur as well. The oxidation half-reaction for zinc has an electrochemical potential of 0,76 V, so it will probably be of influence when gilding brass. Also the carbonates left over from the burnt linen may influence the electrochemical reactions.
In order to establish an environment in which these reactions can take place on the surface of the metal, liquid is required while dissociated salts will help to make the reactions run faster. Van Laer (Van Laer 1721, 166) does not mention the use of any liquid, the other two do.
Research
According to Van Laer, this cold ‘rubbed-on’ gilding technique could be used when a fire gilding left open spots. These could be filled with the cold gilding technique, which was much easier, than the fire-gilding technique, and caused less health problems. After the reproduction experiments, the metal sheets that are plated will be tested. I hope to answer the following questions:
- Can a cold gilding stand high temperatures (annealing/soldering)
- Does it hold when being polished?
- How does it perform during an edurance test?
- Can it stand abrasive materials and tools?
Cross-sections of the gilded copper, brass and silver will be made. With use of a microscope the thickness of the gold layer will be measured and the nature of the binding will be studied. What are the visible differences between fire-gilding, electroplating and “rubbed-on” cold gilding?
Experiments and Results
The experiment took place under primitive conditions, not in an equipped laboratory. I tried recipe 9.1 and 9.2 (Van Laer & I.I.H.); the first experiment did not succeed, because I was not able to dissolve the gold. Raising the temperature may have made the gold dissolve, this was not tested.
Recipe 9.2 was carried out using:
- 12.0 ml HCl
- 36.0 ml H2NO3
- gr. gold (24k, grains (0.1-3.0 mm)
- 44.6 gr. soft linen
- 36.3 gr. rough linen
I used a hammer and a flat stake to flatten the gold grains bigger than 1 mm in diameter. Then I tried to dissolve the gold into 20 ml of Aqua Regia in an erlemeyer. As this did not work, I started warming up the erlemeyer in a bath of warm water of approximately 60°C. I noticed that the gold was etching and the solution becoming yellow. After 30 minutes shaking I added 10 ml Aqua Regia. I did this again after another 45 minutes. Continuously shaking and heating the solution in a warm water bath. After 130 minutes 0.9g. of the gold was dissolved, and a dark yellow solution was formed.
Because everything took place in open air, with only the use of a poor working gas-mask, higher temperatures were not attainable, already a lot of yellow chloride and sulphur fumes could be smelled and where visible. Under safe laboratory conditions higher concentrations of gold in the Aqua Regia are possible to be used.
I distributed the solution equally into 2 erlemeyers. I added the rough linen to one of those, and added the soft linen to the other one. After about 30 hours I took the linen out and allowed it to dry in open air, on a plastic sheet.
After about 6 hours I burned the, not completely dried, linen separately in a clean crucible by putting the crucible in red-hot charcoal, and leaving it standing there for about 1 hour.
The ashes which were produced were pulverised and put into a small container. The ashes produced by the rough linen seemed less heterogeneous both in colour and in particle size than the ones from the fine linen.
Then I tried to gild samples of copper, silver, bronze and brass with the ashes, using a carbonised cork and sputum. The subjective result are presented in Table 1.
| Soft linen | Rough linen | |||
| Amount of effort needed | Visual result | Amount of effort needed | Visual result | |
| Copper | ++ | Good | +++ | Reasonable |
| Silver (92,5%) | + | Good | ++ | Good |
| Bronze | ++++ | Poor/none | +++++ | Poor/none |
| Brass | +++ | Poor | ++++ | Poor/none |
Table 1. (+ fast; +++++ needs a lot of effort)
Best results were produced with the ashes of the soft linen on silver. On bronze and brass it was not possible to create an even layer. I also tried salted water with a cork, a finger and sputum as well as a cotton cloth to gild silver; these methods were less successful. During gilding the polished surfaces became scratched, probably because of hard particles in the ashes as well as in the cork.
Heating gilded silver up to 750°C (temperature for hard soldering. 250°C abbove annealing temperature) destroys most of the visible gold layer, but gilding on the other side of the silver, turned to the stones on which the sample was placed, was not as damaged as the upper part. The disappearing of the goldlayer may have been caused by diffusion effects. At high temperatures thin gold layers may diffuse in the underlayment of silver.
A durability test was carried out by gilding a silver ring with the ashes of the soft linen. Both the inner part and the outer part of the ring were gilded. After about 450 hours of use, the inner part was not visibly damaged, but the outside of the ring showed visible sings of wearing. Scratches through the gold layer where visible, as well as areas where the gold layer was clearly thinner (differences in colour), meaning the layer to be damaged by abrasive attack.
The goldlayer could be easily polished away using a harsch polish on a polishing lathe. Handpolishing with chalk and water did not remove the goldlayer.
Microscopy
Cross-sections of the silver samples and the ring where studied by opticalmicroscopy. Comparing the untreated samples with fire-gilded samples showed that the thickness of the gold layer on the cold gilded samples was about 0.14 times the thickness of a fire gilded gold layer. After etching the samples it became clear that the binding of the gold layer with the silver is strong, because a thin layerof gold-silver alloying is visible. The binding is clearly chemical, not mechanical.
Cross-sections of the heated silver samples showed a gold-layer on one downfaced side and a disturbed surface layer on the other side. It was not possible to determine what this disturbance actually was.
The cross-section taken from the ring showed what was already determined by eyesight, an undisturbed surface on the inner part and a worn surface on the outer part of the ring.
Discussion and Conclusions
A lot of factors were not optimal during the tests and analyses, and could be improved. As test conditions where primitive, the best result possible is probably not reached. Repeated tests in a laboratory will probably give better results. In particular the amount of gold that can be dissolved in Aqua Regia will be higher when working with higher temperatures.
The ashes contained a lot of hard particles. Using different types of linen and a higher burning temperature could improve the quality of the ashes.
The (wine) corks which were used caused a lot of scratches, using a different technique, or other corks, may result in less scratched surfaces.
I did not try to burnish the samples; maybe this could remove a lot of the scratches formed while gilding.
Chemical analyses on samples of ashes, linen and gilded layers where not performed, but could give a lot of information on the mechanism of gilding and could give helpful hints to improve the gilding.
As this cold gilding technique seems to be working well, especially on silver, it is necessary to investigate this technique in a more efficient way. Private, and museum, collections should be studied to find out if cold gilded objects survived time. A more intensive literature study could give more information of the origin of this technique and where it was used in ancient times.
It is possible that this technique can be used as a historical responsible way of repair. I would not go so far to say this could be a technique for restoring. Re-gilding is mostly a repair technique instead of a restoring technique.
Keywords
Country
- the Netherlands
Appendix A
Van Laer`s recipe (Van Laer 1721, 166)
HNO3 (80-85%) 30.8 g.
NaCl 15.4 g.
Au [gold] 1.5 g.
piece of linen
terra cotta pottery
Dissolve the gold in a salt solution in nitric acid. Let the linen take up the solution and allow it to dry. Then burn the linen in a terra cotta pot, or a crucible. To cold gild an object; use a clean piece of linen or a paintbrush. The gold powder is rubbed on the desired places.
I.I.H.`s recipe (I.I.H. 1772-1784, 37)
Aqua Regia (1 part hydrochloric acid (HCl), 3 parts nitric acid (HNO3))
gold
linen
sputum
Dissolve fine gold in Aqua Regia. Wet the linen in this solution and allow it to dry. Then burn the linen to ashes. To gild a piece of silver, clean and polish it thoroughly. Mix some ash with some sputum and rub it on. The join between gold and silver is not as good as with fire gilding.
Höhne`s recipe (Kupferschmiedhandwerk 1839, 371)
Aqua Regia (1 part hydrochloric acid (HCl), 3 parts nitric acid (HNO3))
gold
linen
cork
salt water
This is used for copper, brass and new silver
Dissolve as much gold in Aqua Regia as possible. Wet with this solution fine linen and burn the linen after it has been allowed to dry. The ashes contain very small particles of gold. Red-gold gilding is obtained if 6 parts of gold and 1 part of copper is dissolved in 16 parts of Aqua Regia.
Slightly carbonise the end of a cork in a flame and dip it in salted water. Then dip it in the ashes and rub it on the surface that is to be gilded. Stretch some linen over a cork and use this to polish the gilded surface. One could also use a polishing cloth dipped in soap-water.
Bibliography
BUCHHOLZ-MEISENHEIMER, H., and et al, Ullmanns Encyklopädie der Technischen Chemie, vol. 4., neubearbeitete und erweiterte Auflage, Weinheim, New York, Verlag Chemie, 1986.
DIEBENER, W., Handbuch des Goldschmieds, Ein werkstattbuch für die Praxis, vol. Band III, Stuttgart, Rühle-Diebener-Verlag KG., 1965.
HÖHNE, F., and C. W. RÖSLING, Das Kupferschmiedhandwerk mit den nôthigen Vorlehren über die Erzuegung und Behandlung des ROHKUPFERS, so wie aller in dieses Fach einschlagenden Produkte; Weimar, 1839, Aus “Neuer Schauplatz de Künste und Handwerk”, Hannover, Th. Schäfer, 1839.
van LAER, W., WEG-WYZER voor aankomende Goud en zilversmeden door Willem van Laer, Mr. Zilver-smidt tot Zwoln, Lochem, De Tijdstroom, 1721.
van LAER, W., WEG-WYZER voor aankomende Goud en zilversmeden door Willem van Laer, Mr. Zilver-smidt tot Zwoln, Lochem, De Tijdstroom, 1721.
SCHWAHN, C., Workshop Methods for Gold- and Silversmiths, New York, Chemical Publishing Co., Inc., 1960.
TAYLOR, G., Silver, vol. 2nd edition, Aylesbury, Penguin Books, 1963.

